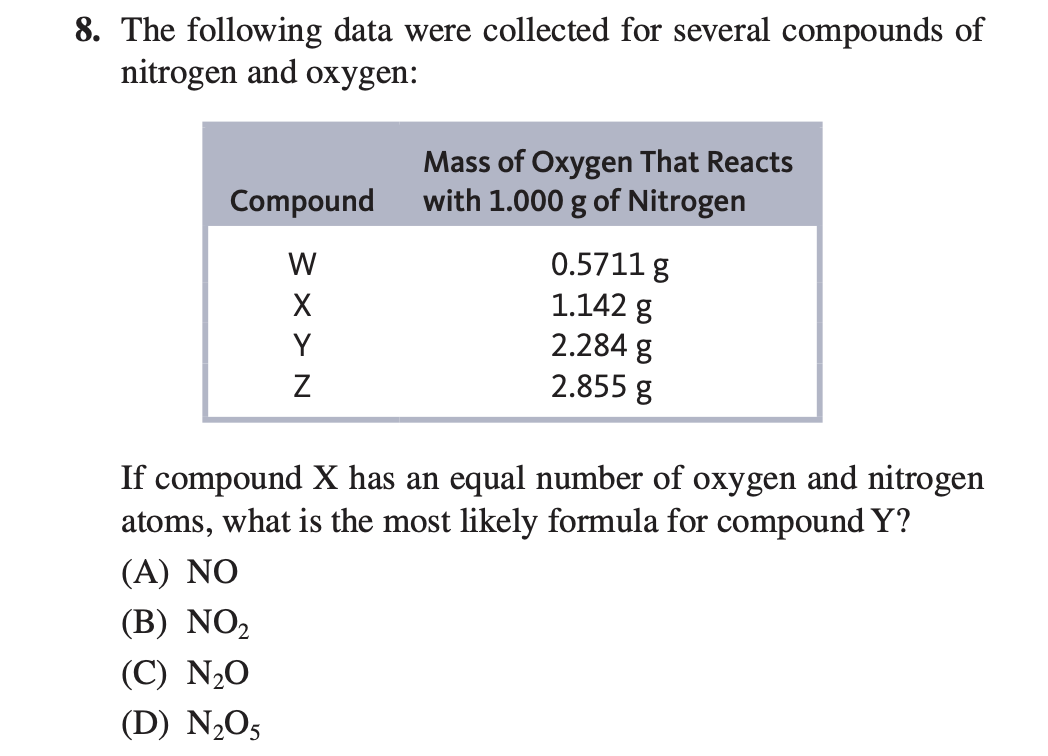
Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen . We have two unknowns x and y. Hydrogen (h 2) has a single bond between.
from www.bartleby.com
the formula mg 2cl 4 has balanced charges with the ions in a 1:2 ratio, but it is not the lowest whole number ratio. There are two types of formulas, empirical and molecular. Calculate the molar ratio of nitrogen to oxygen for each compound.
Answered 8. The following data were collected… bartleby
Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen So, to determine them we need two. the ratio between these elements is 1:1.the formula mg 2cl 4 has balanced charges with the ions in a 1:2 ratio, but it is not the lowest whole number ratio. For every hydrogen atom present in the molecule, there is one oxygen atom.
From www.youtube.com
N2O=N2+O2 Balanced EquationNitrous oxide=Nitrogen+Oxygen Balanced Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen For every hydrogen atom present in the molecule, there is one oxygen atom. There are two types of formulas, empirical and molecular. Calculate the molar ratio of nitrogen to oxygen for each compound. Hydrogen (h 2) has a single bond between. So, to determine them we need two. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.
From www.slideserve.com
PPT Nitrogen Oxides PowerPoint Presentation, free download ID3061496 Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen In ammonia (nh3), the molar ratio of nitrogen to. There are two types of formulas, empirical and molecular. We have two unknowns x and y. A compound was found to contain nitrogen and oxygen in the ratio nitrogen 28 g and oxygen 80 g. So, to determine them we need two. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.
From www.shutterstock.com
Nitrogen Molecule And Oxygen Molecule Stock Vector Illustration Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen Calculate the molar ratio of nitrogen to oxygen for each compound. Hydrogen (h 2) has a single bond between. There are two types of formulas, empirical and molecular. In ammonia (nh3), the molar ratio of nitrogen to.the formula mg 2cl 4 has balanced charges with the ions in a 1:2 ratio, but it is not the lowest whole. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.
From www.researchgate.net
Structures of nitrogencontaining compounds 111. Download Scientific Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen A compound was found to contain nitrogen and oxygen in the ratio nitrogen 28 g and oxygen 80 g. Hydrogen (h 2) has a single bond between.knowing the mass of each element in a compound we can determine its formula. Molecules that contain single, double, and triple bonds. the ratio between these elements is 1:1. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.
From www.numerade.com
SOLVEDTwo samples of different compounds of nitrogen and oxygen have Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen X 2nx2 + y 2ox2 → nxoy. Hydrogen (h 2) has a single bond between. the ratio between these elements is 1:1. For every hydrogen atom present in the molecule, there is one oxygen atom. A compound was found to contain nitrogen and oxygen in the ratio nitrogen 28 g and oxygen 80 g. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.
From www.chegg.com
Solved Nitrogen monoxide and oxygen react to form nitrogen Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen In ammonia (nh3), the molar ratio of nitrogen to. There are two types of formulas, empirical and molecular. Molecules that contain single, double, and triple bonds.knowing the mass of each element in a compound we can determine its formula.the formula mg 2cl 4 has balanced charges with the ions in a 1:2 ratio, but it is. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.
From stock.adobe.com
Elements and Compounds are compared in the molecular structure. Oxygen Which Compound Has The Lowest Ratio Of Nitrogen To Oxygenknowing the mass of each element in a compound we can determine its formula. X 2nx2 + y 2ox2 → nxoy. Molecules that contain single, double, and triple bonds. So, to determine them we need two. We have two unknowns x and y. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.
From www.chegg.com
Solved Nitrous oxide, N2O, has a mass ratio of nitrogen to Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen X 2nx2 + y 2ox2 → nxoy.the formula mg 2cl 4 has balanced charges with the ions in a 1:2 ratio, but it is not the lowest whole number ratio. Molecules that contain single, double, and triple bonds. In ammonia (nh3), the molar ratio of nitrogen to. Calculate the molar ratio of nitrogen to oxygen for each compound. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.
From www.science-sparks.com
What is the Nitrogen Cycle? Science for Kids Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen There are two types of formulas, empirical and molecular. X 2nx2 + y 2ox2 → nxoy.knowing the mass of each element in a compound we can determine its formula. For every hydrogen atom present in the molecule, there is one oxygen atom. the ratio between these elements is 1:1. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.
From www.algaebarn.com
Understanding the Nitrogen Cycle Beginners Education AlgaeBarn Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen So, to determine them we need two. A compound was found to contain nitrogen and oxygen in the ratio nitrogen 28 g and oxygen 80 g. X 2nx2 + y 2ox2 → nxoy. Calculate the molar ratio of nitrogen to oxygen for each compound. In ammonia (nh3), the molar ratio of nitrogen to. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.
From www.bartleby.com
Answered 8. The following data were collected… bartleby Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen In ammonia (nh3), the molar ratio of nitrogen to. We have two unknowns x and y. the ratio between these elements is 1:1.knowing the mass of each element in a compound we can determine its formula. Molecules that contain single, double, and triple bonds. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.
From brainly.in
nitrogen forms 5 compounds with oxygen in which one gram of Nitrogen Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen So, to determine them we need two. Calculate the molar ratio of nitrogen to oxygen for each compound. the ratio between these elements is 1:1. Hydrogen (h 2) has a single bond between. A compound was found to contain nitrogen and oxygen in the ratio nitrogen 28 g and oxygen 80 g. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.
From cartoondealer.com
Nitrogen Oxide Atomic Structure Of Molecule Vector Illustration Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen Hydrogen (h 2) has a single bond between. In ammonia (nh3), the molar ratio of nitrogen to. the ratio between these elements is 1:1. Calculate the molar ratio of nitrogen to oxygen for each compound.the formula mg 2cl 4 has balanced charges with the ions in a 1:2 ratio, but it is not the lowest whole number. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.
From byjus.com
A gaseous mixture contains nitrogen and oxygen in the ratio of 4 1 by Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen For every hydrogen atom present in the molecule, there is one oxygen atom. There are two types of formulas, empirical and molecular.the formula mg 2cl 4 has balanced charges with the ions in a 1:2 ratio, but it is not the lowest whole number ratio. Molecules that contain single, double, and triple bonds.knowing the mass of. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.
From uhighlsu.web.fc2.com
no2 bond order Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen X 2nx2 + y 2ox2 → nxoy. Molecules that contain single, double, and triple bonds. Calculate the molar ratio of nitrogen to oxygen for each compound. In ammonia (nh3), the molar ratio of nitrogen to. For every hydrogen atom present in the molecule, there is one oxygen atom. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.
From classnotes.org.in
Oxides of Nitrogen Chemistry, Class 12, The pBlock Elements Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen So, to determine them we need two. X 2nx2 + y 2ox2 → nxoy. Molecules that contain single, double, and triple bonds. Hydrogen (h 2) has a single bond between.the formula mg 2cl 4 has balanced charges with the ions in a 1:2 ratio, but it is not the lowest whole number ratio. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.
From socratic.org
How are elements and atoms related? + Example Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen the ratio between these elements is 1:1. Molecules that contain single, double, and triple bonds.the formula mg 2cl 4 has balanced charges with the ions in a 1:2 ratio, but it is not the lowest whole number ratio. Hydrogen (h 2) has a single bond between. We have two unknowns x and y. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.
From www.britannica.com
Nitrogen Definition, Symbol, Uses, Properties, Atomic Number, & Facts Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen X 2nx2 + y 2ox2 → nxoy.the formula mg 2cl 4 has balanced charges with the ions in a 1:2 ratio, but it is not the lowest whole number ratio. Calculate the molar ratio of nitrogen to oxygen for each compound. We have two unknowns x and y. There are two types of formulas, empirical and molecular. Which Compound Has The Lowest Ratio Of Nitrogen To Oxygen.